Calculation of NOx formation using the detail kinetic
mechanism
Combustion of complex hydrocarbons occurs by stages:
-
the first stage is very fast disintegration of a fuel molecule
on radicals and molecules with smaller number of atoms;
-
further there is considerably slower process of delayed burning
in which particles with one - two atoms of carbon participate.
For simplification of the description of the first stage of
combustion for more complex, than methane, hydrocarbons (components of diesel
fuel, biofuel, DME) gross-reactions which describe disintegration of the higher
hydrocarbons and their derivatives up to simple molecules and radicals are
entered.
Initial disintegration kinetic of a fuel molecule is described by 40 reactions
with participation of 10 species.
It is supposed, that regardless of initial composition of hydrocarbon at its
combustion in the second phase participate the same simple hydrocarbons.
There-fore for the description of delayed burning process non-empirical detail
kinetic mechanism of combustion of the elementary hydrocarbon – methane is used.
The kinetic scheme of NO formation at combustion of the methane is com-piled on
the basis of the kinetic scheme by prof. Basevich V.J.:
CH4 + O2 Û
CH3 + HO2
CH3 + O Û H + H2CO
. . . . . . . . . . . . . . . . . .
CH + N2 Û HCN + N
N + O2 Û NO + O.
The given scheme consists of 199 reactions and determines
concentration of 33 species: CH4, C2H, C2H2,
C2H3, C2H3O2, C2H4,
C2H5, C2H6, CH, CH2, CH3,
CH3O, CH2O, CHO, CO, CO2, H, H2, H2O,
H2O2, HO2, O, O2, OH, N2,
N, NO, NO2, N2O, HNO, NH, HCN, CN.
The material balance of the species participating in chemical reactions, is
described by system of the kinetic equations:
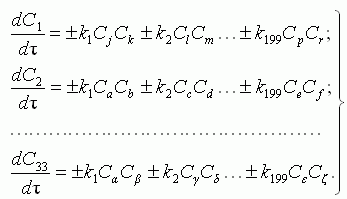 , ,
where: Cj, Ck ,… Cζ are mole fraction
of the appropriate substances; τ is time; ki are the
constant of chemical reaction rate, determined on Arrhenius equation:
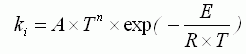 , ,
where: A is pre-exponential factor, n are an exponent, Å
is energy of activation, R is absolute gas constant.
The numerical solution of the differential equations system is carried out by
Gear’s method with variable order of accuracy.
To page "Calculation
models"






|



