Simulation of Nitrogen Oxides formation in cylinder of ICE
Dr., Professor Vasiliy Zvonov, Dr. Andrey Kozlov
At simulation of nitrogen oxides formation it is
accepted that the cylinder is divided into two zones: zone of fresh charge and zone of
burnt gas. Zone of fresh charge consists of air, fuel and residual gas. Before combustion
fresh charge zone is alone. During combustion a volume of burnt gas zone increases. At
calculation of combustion there is an assumption, that a local air-fuel equivalence ratio
at combustion varies linearly from initial value AFini < 1 up to 1.
Current value of air-fuel equivalence ratio at combustion AFc is a
function of crank angle f:
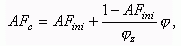
where: fz is a combustion duration, CA.
Features of a designed procedure are:
- Step by step calculation of equilibrium composition of combustion products for eighteen
species in the burnt gas zone./1/
- Kinetic calculation of thermal nitrogen oxides formation with chain Zeldovich mechanism
/2/.
As for requirements of combustion of propellants in explosion engines
defining the formation "thermal" NO is, in tendered model all calculations are
yielded on the thermal mechanism.
Because in engine a "thermal" NO are main, in
simulation model all calculations are carried out with thermal mechanism.
The oxidizing of nitrogen be on the chain mechanism, basic
reactions are:
O2 <=> 2O;
(1)
N2 + O <=> NO + N;
(2)
N + O2 <=> NO + O.
(3)
A main reaction is 3. Rate of this reaction
depends on concentration of atomic oxygen.
The calculation of NO formation with equation of
the chain mechanism is carried out for the zone of combustion, then the medial NO
concentration over whole combustion chamber is determined. Volume concentration of NO in
combustion products formed in a current calculation step is defined with equation:
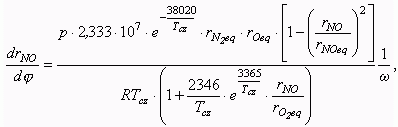
where: p is a cylinder pressure, Pa;
Tcz is a temperature
in a burnt gas zone, K;
R is a gas constant,
J/(mole K);
w is an
angular crank velocity, 1/sec;
rNO eq, rN2 eq,
rO eq, rO2 eq are equilibrium concentrations
of an oxide of nitrogen, molecular nitrogen, atomic and molecular oxygen, accordingly.
The equilibrium concentrations of 18
species are calculated on every time step. List of species includes:
O, O2, O3, H, H2, OH, H2O, C, CO, CO2,
CH4, N, N2, NO, NO2, NH3, HNO3,
HCN.
Overall system of equations includes: 14 equilibrium equations, 3 equations of material
balance, and Dalton equation.
NO concentration in a cylinder:
rNO c = rNO rbc,
where: rbc is a fraction of burnt gas in a cylinder.
NO concentration in a "dry" burnt
gas of a cylinder: rNO dry = rNO / (1 - rH2O),
where: rH2O is a volume fraction of water vapor in a
combustion chamber.
Specific NO emission, g/kWh:
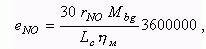
where: Мbg is a mass of burnt gas in a cylinder at
the end of combustion, kmole;
Lc is working
cycle work, kJ;
hм
is a mechanical efficiency of engine.
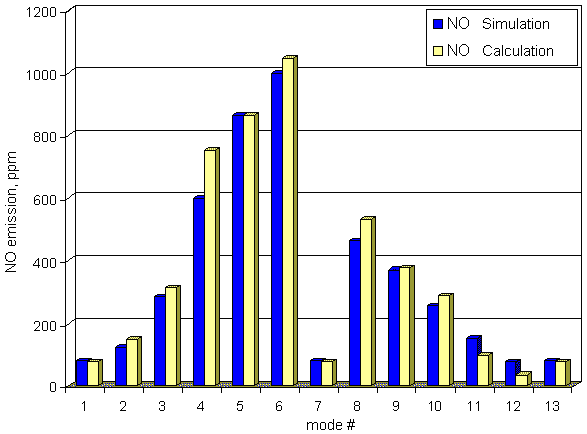
Comparison of calculated NO emission with
measured ones of truck diesel YaMZ-7512 working on 13 modes cycle is presented below.
Bibliography

To page "Calculation
models"






|



